E3 MED
Transporting a package containing infectious substances between its point of origin, transport vehicles, warehouses, and its destination can pose difficulties due to movement, vibrations, and variations in temperature, humidity, or pressure. It is therefore essential that the packaging used to transport infectious substances be of good quality and sufficiently sturdy to withstand the conditions it may encounter. This is why various standards and regulations have been imposed for their transport and packaging.
With the Diagnobox packaging range, E3 Cortex offers products that are perfectly designed to facilitate the shipment of potentially infectious Class 6.2 Category B (UN3373) samples. They comply with current standards (IMDG, IATA, RID, ADR) and offer customizable labeling options depending on the quantities.
E3 MED
Diagnobox for the transport of infectious substances in class 6.2
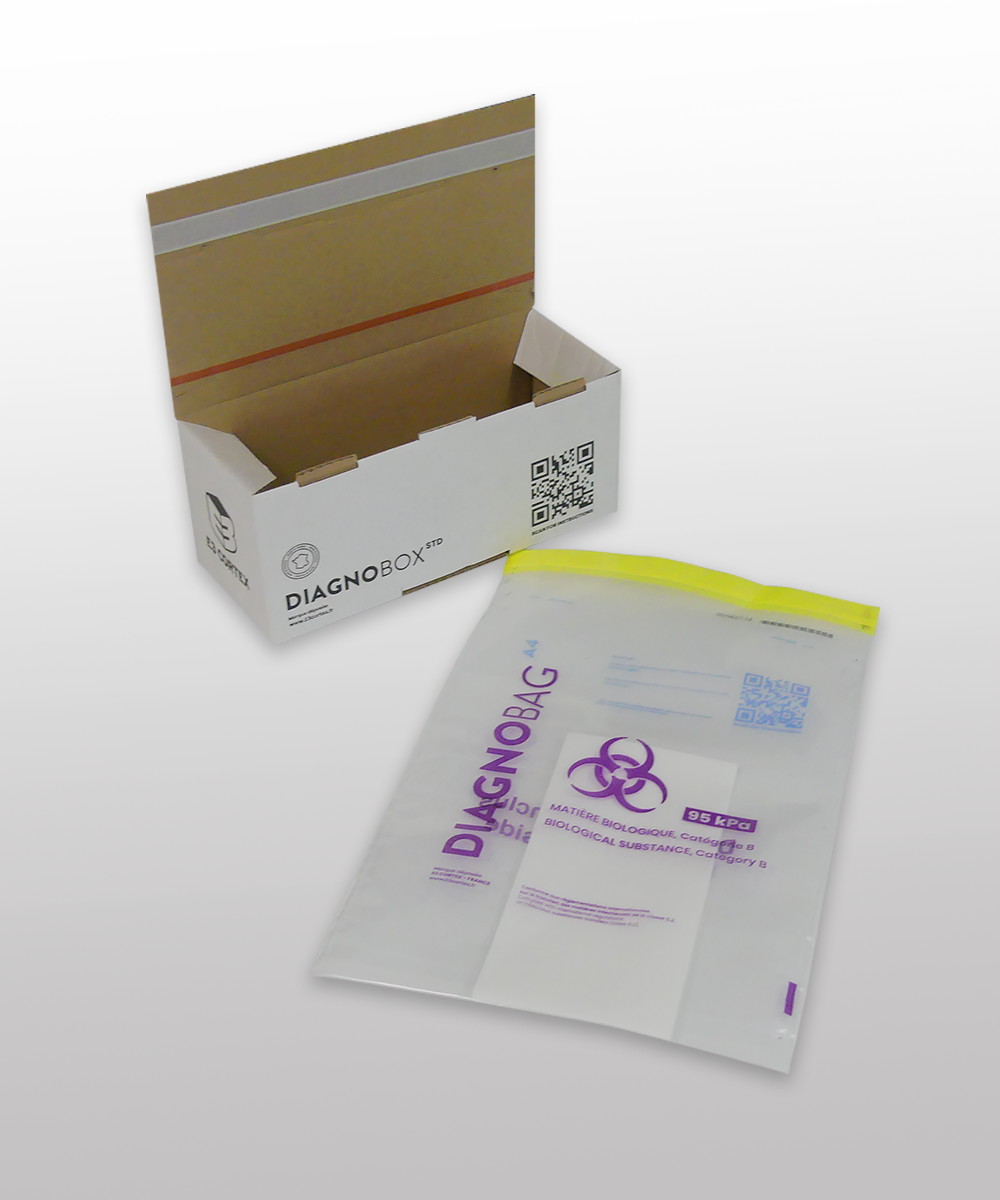
Diagnobox is a specific packaging for the transport, by land or air, of diagnostic samples and products belonging to class 6.2 (infectious substances) and listed under UN code 3373 Biological Material, Category B. It is a packaging used as part of the triple packaging required by current regulations.
Infectious substances are subclassified in Category B when they potentially contain biological agents that could cause infection in humans or animals, but do NOT meet the criteria for Category A; that is, the consequences of infection are not considered to be seriously debilitating or potentially fatal.
Packaging used to ship infectious substances must consist of triple packaging, namely a leak-proof primary receptacle, leak-proof secondary packaging, and tertiary packaging protecting the secondary packaging and its contents from external damage (impact, water, etc.) during transport. This type of packaging must undergo multiple tests and checks to ensure it meets specific resistance standards.
The purpose of this triple packaging is, of course, to protect the primary container (tubes, vials, etc.) so that it arrives at its destination undamaged, but also to protect people and the environment from any risk of contamination.
E3 MED
Diagnobox packaging allows you to transport your products and materials safely.
Made from double-sided, small-flute cardboard, Diagnobox has an automatic bottom, a Kraft inner cover, and a white outer cover. The markings on this box are defined by current regulations (ADR, IATA).
It is a single-use packaging with a tamper-proof system to protect samples from any attempts at theft or exchange. This guarantees the sender that the sample received by their customer is indeed the one that was sent.
The Diagnobox range consists of UN3373 printed packaging, depending on the model. Designed in accordance with ADR P650 and IATA 650 instructions, Diagnobox packaging must be used with one or more secondary packages, which themselves contain one or more primary packages containing the samples, such as the Diagnobag packaging from our product range. The primary or secondary packaging must be resistant to a pressure differential of 95 KPa and certified by an approved laboratory. Diagnobox is delivered flat and can be assembled instantly with the Diagnobag A5 plastic bag and absorbent material as required.
Diagnobox is particularly suitable for transporting serology samples in organ perfusion machines. The Diagnobox range can be customized according to the quantity ordered. Its very small footprint makes it an economical product.








 Technical specifications
Technical specifications 